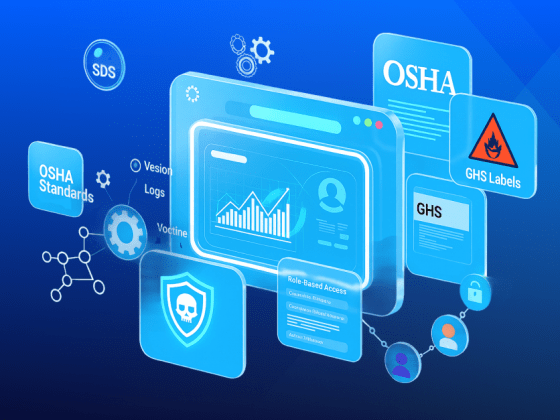
Integrated Chemical Management (iCM) is an industry-first solution, fully-integrated with Microsoft Dynamics 365 Finance & Supply Chain
iCM is specifically tailored to meet the needs of the following industries
Coating
Contract Manufacturing (CMO, CDMO)
Biotech
Chemicals
Bulk / Commodity Chemicals
Specialty Chemicals
Life Science
Pharmaceutical Manufacturing
More

SDS Management
- SDS authoring, maintenance, and approvals
- Automated workflows
- Create and maintain all master data elements for SDS and label printing
- Multi-language support (all languages supported by Microsoft)
- SDS templates
- SDS versioning by country and language
- SDS distribution for customers, contacts, and employees
- Full audit management and changelog
- Integration with select 3rd party chemical compliance software, such as LISAM, 3E, etc.
- Configurable role-based security and permissions
- Many more features
Label Management
Efficient Label Printing with Reusable Templates and Automated Workflows
- Label definition and setup
- Reusable label templates
- Leverage regulatory data for label printing
- Print labels via automated workflows for key operations such as receiving, shipping, production, quality, internal labels and more
- Single and multi-language labels (All language supported by Microsoft)
- Role-based security and permissions
- Full audit management and label logs
- Commonly used labels, including DOT labels, raw material labels, shipping labels, finished goods labels, lab labels, labels for hazardous and non-hazardous goods
- Ad-hoc labels of any kind, and more.


Other Features
Streamlined DEA Management and E-Signatures for Safe and Quality Orders
- DEA management – holds on sales orders
- Electronic signatures on quality orders, routes cards, receipts, etc.
- Physical and chemical properties, and other iCM elements for raw materials, finished goods, formulas, and more.
Want to learn more about Integrated Chemical Management (iCM)?
iCM FAQ’s
-
01.What is iCM?
Integrated Chemical Management (iCM) is the ultimate solution for businesses operating in the chemical industry. Built on the powerful Microsoft Dynamics 365 Finance and Operations platform, iCM brings together multiple critical functions into a single, comprehensive application.
With iCM, you can effortlessly manage your SDS and label management needs, including private labels, ensuring that your products meet all necessary regulatory requirements. The platform also offers robust DEA management features, enabling you to monitor and track controlled substances with ease.
iCM is designed to simplify your processes, enhance compliance, and increase productivity, allowing you to focus on what matters most - growing your business. As a Microsoft Preferred solution, you can trust that iCM is built on top of cutting-edge technology and backed by world-class support.
-
02.What features are included for SDS Management?
- DEA management – holds on sales orders
- Electronic signatures on quality orders, routes cards, receipts, etc.
- Physical and chemical properties, and other iCM elements for raw materials, finished goods, formulas, and more.."SDS Authoring & Approval with workflows
- SDS Maintenance (create and maintain SDS Setups)
- SDS Distribution (Multiple items, Customers and Contacts)
- Note – End-to-end workflows have been built and enabled for Authoring, Maintenance & Distribution functions.
- Create and maintain all master data elements related to SDS’s
- SDS Templates
- SDS Versioning (Maintenance of major and minor versions)
- Maintain SDS Versions by Country & Language
- Multi-language support for all languages supported by Microsoft
- Auto-derivation rules to derive GHS Label elements by GHS Version
- Note – Auto derivation rules are currently available for Section 2 of the SDS. Automated derivations for other viable sections are in pipeline and will be available in future releases.
- Ability to store and print pictograms
- SDS Item Groups – Ability to link multiple items to a single SDS
- Ability to print SDS with your customer’s logo on it (private labels)
- Everything SDS (Logs, Workflow configurations etc.)
- Enhanced logs for SDS Master, Labels and Label Printing
- Mass updates of Safety Data Sheets with any statement or elements changes such as precautionary statements, hazard statements, signal words, pictograms etc.
- “Mass Activation” and “Mass Deactivation” of SDS
- Role-based security for key functions within SDS Management"
iQM provides extensive functionality in CAPA management, allowing you to easily and efficiently manage corrective and preventive actions. The platform seamlessly integrates with Microsoft Dynamics 365 Finance and Operations, offering streamlined workflows and the automation of manual processes. iQM is designed to meet the unique needs of the quality management industry, providing a user-friendly and customizable interface to tailor the solution to your specific business requirements.
-
03.What features are included for Label Management?
- Label definition & setup
- Label printers setup
- Print labels via updated & automated workflows –
- Receiving
- Shipping
- Production (incl. Packaging)
- Quality
- Transfer orders
- Print Adhoc labels
- View label log
- iCM Administrator functions
- Group label printing
- Group label printing to multiple printers
- Multi-tray label printing
- Set print destinations on label definitions
- Placeholders for certification images such as kosher, halal, etc.
- Language selection for SDS sections such as; hazardous statements, precautionary statements, etc.
- Additional physical properties such as; specific gravity
- Finished goods label printing – by customer
- Set print destination settings on label print form
- Label printing option included on shipments screen
- Workflows for label creation & setups
- Print finished goods label on packing slip transaction, fg labels are now included in the post packing slip window
- Label printing with storage, tracking and product dimensions
- & more.
-
04.What other features are included with iCM?
DEA functionality
-
05.Who can use iCM?
- Anyone that requires the ability to view or update Chemical / Regulatory data of an item or formula in their ERPs Product Information Management (PIM) module.
- Anyone that defines and configures labels for different transactions
- Anyone that prints labels in the back-office or on the shop-floor
- Anyone that maintains chemical regulatory data
- Anyone that authors, views or distributes Safety Data Sheets (SDS) or related information
- Anyone that works with DEA customers or contacts
-
06.Are these products available in my country?
Just because we’re based in Chicago doesn’t mean our products only work in North America - Quality management and compliance are important everywhere, especially when it comes to highly-regulated industries, and our products are available for dozens of countries around the world. Get in touch with us today to make sure our products support you!
-
07.How much do these solutions cost?
"Making sure our customers are satisfied is a top priority, no matter what services or solutions they require. We offer competitive pricing on our core offerings, along with simple licensing plans for businesses of all sizes. Contact us today for more information.
-
08.We can’t decide what system will work best for us.
You’re not alone. In fact, this is one of the main reasons most businesses end up with ‘aging legacy software’. Thankfully that’s where we come in - our experience with implementations for regulated industries can help fast track your decision, making sure you don’t skip a beat.
-
09.I have additional questions, where can I find more information?
Want to learn more? Contact us today to see how we can help you achieve your goals.
Our InsightsSee All
How to Manage Supply Chain Disruptions: Causes and Impacts
How to Manage Supply Chain Disruptions: Causes and Impacts https://xcelpros.com/wp-content/uploads/2021/11/How-to-Manage-Supply-Chain-disruptions-card-thumbnail.png 1108 1008 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gPost COVID19, most sales and marketing teams have gone remote. The unforeseen circumstances of not having an in-person interaction with customers have driven businesses to invest in the latest and conversion-oriented CRM tools.
How technology helps in optimizing the Pharmaceutical supply chain
How technology helps in optimizing the Pharmaceutical supply chain https://xcelpros.com/wp-content/uploads/2025/12/pharmaceutical-supply-chain-role-of-technology-right.jpg 1500 1071 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gThe inability to adapt to technological advancements and rapidly changing regulatory landscape makes managing pharmaceutical supply chains quite a tough pill to swallow. Successful pharmaceutical companies leverage the power of IT to streamline their supply chain operations.
On-Time Delivery (OTD) KPI Your Most Important Metric In Operations Management
On-Time Delivery (OTD) KPI Your Most Important Metric In Operations Management https://xcelpros.com/wp-content/uploads/2019/01/on-time-delivery-in-operations-part-2-banner-right.jpg 800 571 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gAt a Glance: Why On-Time Delivery (OTD) Matters According to Harvard Business Review research, increasing customer retention rates by just 5% can increase profits by 25% to 95%, highlighting the critical importance of meeting customer demands on time. A 2024 industry report showed that 69% of consumers are less likely to shop with a retailer…
Batch Traceability in Pharmaceutical Manufacturing
Batch Traceability in Pharmaceutical Manufacturing https://xcelpros.com/wp-content/uploads/2025/10/batch-traceability-in-pharmaceutical-manufacturing-right.png 950 627 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gThe pharmaceutical industry has faced many challenges. One of the biggest challenge is how to address batch traceability when it comes to pharmaceutical manufacturing.
Streamline Your Supply Chain with Advanced Warehouse Management System
Streamline Your Supply Chain with Advanced Warehouse Management System https://xcelpros.com/wp-content/uploads/2025/10/streamline-your-supply-chain-with-advanced-warehouse-management-system-right-final.jpg 950 627 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gDiscover the capabilities that D365 Advanced Warehouse Management brings to the table to address key warehouse management challenges.
Pharmaceutical Manufacturing Cost Breakdown: Why It Matters
Pharmaceutical Manufacturing Cost Breakdown: Why It Matters https://xcelpros.com/wp-content/uploads/2021/09/pharmaceutical-manufacturing-cost-breakdown-right.jpeg 800 528 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gForecasters are projecting more opportunities for mid-market pharmaceutical companies operating in the US. These opportunities will require more streamlined processes within pharmaceutical manufacturing and contract development and manufacturing organizations (CMOs and CDMOs).
Cybersecurity Compliance in Chemical Plants: Managing Rising Risks
Cybersecurity Compliance in Chemical Plants: Managing Rising Risks https://xcelpros.com/wp-content/uploads/2025/09/cybersecurity-compliance-in-chemical-plants-right-img.png 950 627 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gDigital security threats are increasingly targeting industries—including the chemical sector—to the point the Department of Homeland Security is now issuing cyberterrorism guidelines.
Managing Chemical Compliance With ERP: OSHA to GHS
Managing Chemical Compliance With ERP: OSHA to GHS https://xcelpros.com/wp-content/uploads/2025/09/managing-chemical-compliance-with-erp-osha-to-ghs-right.png 950 627 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gWhen used effectively, modern enterprise resource planning software (ERP) can help companies in several ways when it comes to chemical regulations
How Technology is Solving Pharmaceutical Compliance Risks in 2025
How Technology is Solving Pharmaceutical Compliance Risks in 2025 https://xcelpros.com/wp-content/uploads/2025/09/how-technology-is-solving-pharmaceutical-compliance-risks-in-2025-right.png 950 627 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gMany pharmaceutical companies have restructured their financial and resource allocation models to invest more in adhering to compliance. Compliance requirements around the globe have grown in past decades.
Audit Readiness in Pharma: From Scrutiny to Systemic Strength
Audit Readiness in Pharma: From Scrutiny to Systemic Strength https://xcelpros.com/wp-content/uploads/2021/01/828blogbannerRight.png 950 658 Xcelpros Team https://secure.gravatar.com/avatar/eb2f8c285d005e0a1f2e0e90930b154a89fb9ffee085d55d1ee27cf29815eff7?s=96&d=mm&r=gThe pharmaceutical market has faced various challenges during COVID-19, including a complex set of regulatory standards. Due to the stringent FDA pharmaceutical regulations, pharma companies always need to keep up with managing compliance tools.