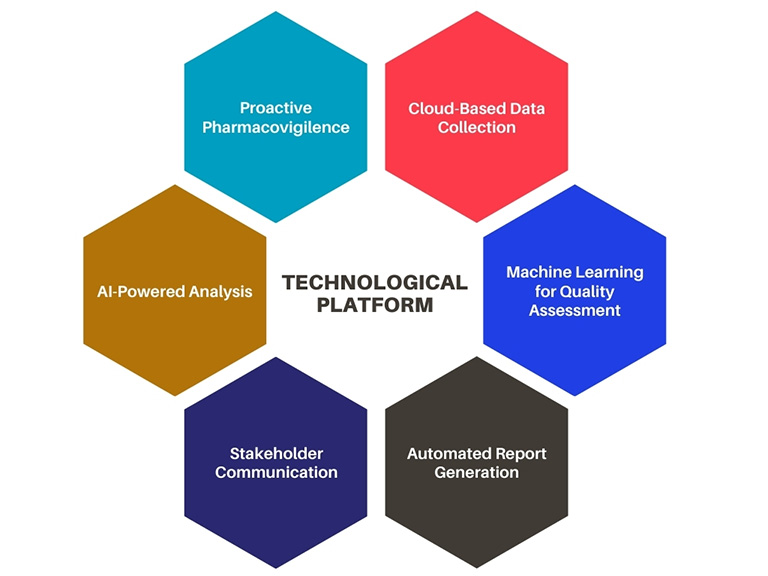
At a Glance
The pharmaceutical industry faces some unique warehouse management challenges. Many of these issues can dramatically impact medications, even though they may not exist in industries such as general retail.
Key issues facing warehouse managers include:
- Keeping portions of their facilities at the correct temperatures to prevent medications from spoiling.
- Following federally-mandating good manufacturing process rules.
- Security issues for products and intellectual property.
- Inventory controls.
Warehouse issues specific to the pharmaceutical industry include:
- Temperature control: Active pharmaceutical ingredients (APIs), precursor chemicals, and manufactured drugs frequently require controlled temperatures. A general temperature range for a cool, dry place is between 59-77° F (15-25° C). Some products, such as vaccines, may require freezing. Exposing drugs to the temperature outside their effective ranges can cause chemical changes and reduce a drug’s effectiveness. For example, Baystate Health states that medications containing hormones do not work as well when exposed to colder or hotter temperatures.
- Humidity control: Moisture condensing inside packages can impact a medication’s effectiveness. Baystate Health states that blood glucose strips exposed to humidity will give inaccurate readings.
- Light exposure: Exposure to ultraviolet light from the sun and other sources can change the chemical structure of some medications. The light exposure causes photodecomposition, reducing the medication’s potency. Light exposure can also cause side effects after administration, such as phototoxicity and photoallergy, a 1997 post in PubMed states.
- Adhering to the Food and Drug Administration (FDA) Current Good Manufacturing Process (CGMP) standards for warehouses, processes, and drugs: Includes keeping careful track of item locations within the warehouse.
According to Kanban, the FDA’s CGMP warehouse standards include the following:
- Contamination prevention: Storage must allow inspection and cleaning.
- Identification: Each drug must have a unique, traceable code that identifies the lot’s status, such as approved, quarantined or rejected.
- Distribution Procedures: Written procedures describing the distribution process for each drug including recalls.
- Storage Procedures: Written procedures describing the storage conditions for each drug are required.
Some pharmaceuticals require only temperature controls for specific ranges. Other medications require climate-controlled environments affecting temperature and humidity.
Figure: 1Pharmaceutical Warehouse Management Challenges
Planning on transforming your pharmaceutical warehouse to get over operational challenges? Get started with a assessment!
Following GMP Rules
GMP SOP states that following the Good Manufacturing Process (GMP) rules enables manufacturers to:
- Protect medicines and raw materials for medicines during storage
- Prevent finished product degradation
- Avoid contamination from other materials
- Prevent damaged or expired products from being shipped
Warehouse managers also face the challenge of keeping track of three types of items appearing on the packaging bill of materials governed by GMP procedures. Each of these item types requires unique lot numbers:
- APIs, precursor chemicals and other starting materials
- Packaging materials
- Printed materials
All warehouse managers face inventory control requirements. Those in the pharmaceutical sector also deal with intense government scrutiny.
Receiving Shipments
Other GMPs in the pharmaceutical industry require materials arriving from suppliers to be reviewed based on their use. For example, it’s important to check starting chemicals to confirm they are:
- From a source approved by the company
- Free of damage and defects
- Labeled with all required information
- Have a unique identifier
- Registered in the company’s inventory database
- Quarantined until quality control tests are performed
- Stored appropriately and safely, such as in a temperature-controlled section or “Dangerous Goods” area for flammable and toxic materials
Unlike retail goods warehouses, pharmaceutical warehouse managers should also set aside an area for raw materials to be tested and confirmed to meet all required standards. A similar section should exist for any materials that fail these tests, GMPSOP states.
Sampling and Testing
Sampling and testing should be done in a room having sections with positive air pressure (i.e., the air pressure is higher than that outside, preventing contaminants such as dust, microbes, pollen, cleaning agents and lubricants from entering) and negative air pressure (i.e., the pressure is lower than that outside to prevent materials from inside the room going outside). An airlock with positive pressure keeps out external contaminants. With the airlock sealed, the inner testing can have negative air pressure to keep chemicals from contaminating the larger warehouse.
Other sampling room requirements include clean instruments and appropriate personal protective equipment (PPE) as required by the federal Occupational Health and Safety Act (OSHA) and the FDA. OSHA has a downloadable brochure on warehouse safety.
Storage and Tracking Inside the Warehouse
“Lack of control over material movement in the warehouse can, and has, led to defective products,” GMPSOP states.
General warehouse practices (GWP) require that:
- Received unused goods and finished products are quarantined until approved for release.
- Items have correct status labels (e.g., current, expired, etc.)
- Unique identifiers are visible.
- Products are stored by type when appropriate.
- Access to toxins and addictive drugs or chemicals is stored separately. Access is limited only to approved personnel.
- Materials are tracked as they move through the production facility from the Receiving area to Production and then to the Shipping.
When possible, warehouse managers should have separate sections to store damaged or returned goods, recalled items, “not for sale” samples and when identified, counterfeit materials.
Labeling
Another challenge for pharmaceutical warehouse managers is accurate labeling. GMP rules require labels to include a familiar name and Unique Identification Number that must be different from the supplier’s lot number. The UIN must be recorded in the lab, on the facility’s computer system, and in production. GMP SOP suggests not referring to the IUN as a batch number.
Other requirements unique to pharmaceutical labeling include:
- Expiration dates
- Barcodes for additional tracking options
- Status indications, typically in the form of a color code
- Quarantined products
- Items being held for investigation
- Rejection labels when an item fails to meet required standards
- Approval and/or release labels indicating the item can proceed to the next step in the supply chain
Security Challenges
Medicines and other pharmaceutical products are in high demand, making them tempting targets.
Warehouses should have secure physical storage areas for raw materials and finished products.
In addition, Avcostar states that the formulary, drugs, and drug components are expensive and prone to theft. It suggests performing a risk analysis audit that includes where known security breaches occurred. “The company can then focus on identifying and eliminating the most vulnerable posts and systems against malicious access, modification or deletion of data, enhance access control to systems and data and implement new cybersecurity best practices,” Arecont Vision Costar VP of Marketing Jeff Whitney states.
The code of federal regulars 21 CFR Chapter 1 requires control of all production stages, including system validation and audit trails. Refer to this article from Cornell Law School for detailed information.
Solving Challenges
Effective use of warehouse management computer systems, such as the warehouse management module in Microsoft Dynamics 365’s Supply Chain Management, can help track inventory management in pharmaceuticals accurately and manage these challenges.
The module “has a wide range of features to support the warehouse facility at an optimal level at any time,” according to Microsoft. Among the warehouse module’s functions are:
- Workflow support
- Using mobile devices
- Full batch and serial item support
- Label printing and routing