At a Glance
- The World Health Organization (WHO) emphasizes providing the most effective drugs that do not cause severe adverse effects.
- Understand adverse effects and how to prevent them by assessing the right chemicals, quantities and processes in medical vaccine manufacturing.
- Digital solutions and services are designed to help scientists and pharmaceutical company decision-makers detect, assess, understand and prevent adverse effects from their medicines.
- Starting now, pharmaceutical companies are placing a greater emphasis on drug quality checks to avoid mistakes caused by rushing the production process.
Introduction
Today, most people are familiar with terms like “clinical trials,” “safety assessment tests for vaccines” and “FDA approvals” because of COVID-19 media coverage over the last few years. While these terms have always been a part of any pharma and biotech manufacturing companies’ quality assurance program, the ongoing pandemic has made them part of everyday conversation.
People often wonder why it takes so long for a drug or vaccine to get approved for mass use, and rightfully so. The answer however, lies in the principles of pharmacovigilance, also known as drug quality control.
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem.
The Primary Goals of Pharmacovigilance Guidelines
The goals of a typical pharmaceutical quality control program include:
- Assessing a drug’s short-term and long-term adverse effects and any harm the drug might cause a patient.
- Continuously collating and monitoring a particular drug’s safety data.
- Assessing the risks and rewards of the drug to make a guided decision on the administration of the drug.
- Communicating adverse drug reactions (ADRs) data between health professionals and clinical researchers while maintaining transparency at all levels.
- Preventing the distribution and administration of unsafe drugs by medical bodies and drug companies.
All pharmaceutical companies require a team of professionals to carry on this constant quality check of their drugs. This team can include scientists, clinicians, biochemists, physicians and medical writers. The team’s job is to collect, collate, analyze and assess the safety profile of every drug.
This task requires constant alertness and unprecedented agility for an accurate and quick response. In today’s market, manual data reviews are no longer adequate. Combining finely tuned digital tools and well-trained employees is the best way to protect patients from severe injury or death.
A drug maker’s automated quality control program should be smart enough to help collect, analyze and check data. The software equipped with artificial intelligence can check a drug’s composition, verify safety profile mapping and perform other crucial steps required for necessary quality checks.
Pharmaceutical companies need to follow a wide range of procedures to ensure their pharmacovigilance is up to government and industry standards in order to remain compliant.
“The scope of the problem of poor-quality drugs transcends national borders because the manufacturing and supply chain of medical products thrives in an international market.” Elizabeth Ndichu, MD, and Kevin Schulman, MD
Book a consultation learn how technology can simplify your pharmacovigilance needs.
To comply with new quality standards and still meet today’s demands for fast-track drug development and clinical trials, pharmaceutical and biotechnology firms need to strictly adhere to the process of end-to-end pharmacovigilance.
- Carrying out detailed patient surveys for different age groups, countries and health conditions
- Using consultants and experts to manage its quality control program
- Using software such as Microsoft Dynamics 365 for the pharmaceutical industry to help ensure error-free data maintenance, analysis and report generation
- Keeping all stakeholders in loop at all times to avoid any errors
- Maintaining records of their quality control policies and procedures. These records are required for medicines plus other products such as cosmetics, nutritional supplements and dietary products
- Formulating a plan for intervention, mitigation, assessment and resolution in the event of drug quality issues
Figure 1:Digital Ecosystem: Pharmacovigilance
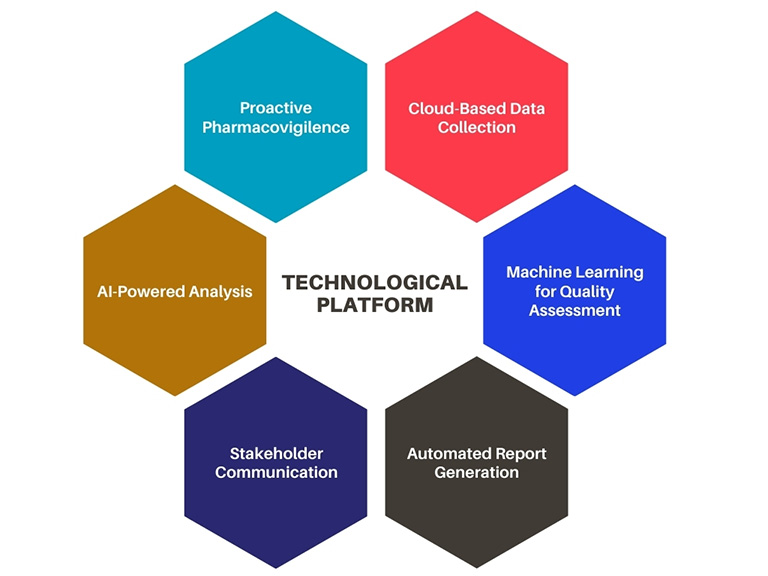
Pharmaceutical companies need to take an agile approach toward pharmacovigilance. Leveraging technology for streamlining safety procedures and quality checks is no longer a matter of convenience but a necessity.
Solutions like Microsoft Dynamics 365 Finance and Supply Chain Management provide Quality Control functionality. Each of these computer programs has methods to collect, track and report quality test results. This software is a comprehensive solution that makes it easy to leverage technology to streamline quality check operations.
These solutions can pave the way towards regulatory compliance, stringent component mapping and monitoring of a drug’s safety profile. It reduces manual intervention by employees, allowing individual case safety reports (ICSRs) to be performed easily.
Microsoft Dynamics modules can be used by different departments by the likes of clinical researchers, scientists, medical writers, physicians, medical representatives and government drug governing bodies. These solutions for monitoring drug safety are considered one of the best investments a company can make today.
Key Takeaways
- Technologically-enhanced pharmacovigilance is the need of the hour for today’s pharmaceutical companies.
- The pharmaceutical sector continues to evolve on a large scale. This change requires gathering medicinal data at a global level to map drug safety.
- Forming a blueprint to follow the pharmacological journey is a critical step for any pharma and biotech company.
References: Pharmacovigilance: Regulation and Prequalification







