2025 Perspective: What experts across pharma ops, analytics, and compliance now say about visibility
In 2025, supply chain visibility is no longer just a support role – it’s a core capability for pharmaceutical companies. With tighter regulations, higher patient expectations, and more complex global networks, clear, end-to-end visibility is essential to stay compliant and competitive.
Four years ago, discussions around supply chain visibility focused on inventory accuracy and compliance checklists. But the conversation has matured. In today’s pharmaceutical ecosystem, visibility now refers to how clearly, quickly, and confidently an organization can understand what’s happening across sourcing, production, distribution, and delivery. And just as importantly – how soon they can act on that insight.
Visibility Isn’t a Tool – It’s a Capability
Visibility is often seen as just software or reporting, but leading pharmaceutical companies recognize it as a foundational operational competency embedded across systems, processes, and people. Without it, even the most sophisticated supply chains miss early warning signs, resulting in delays, quality issues, and escalating risks.
Breakdowns typically occur in the middle layers of the supply chain; Tier-2 and Tier-3 suppliers, regional warehouses, and handoffs between logistics partners. These gaps aren’t caused by negligence but stem from tribal knowledge, outdated systems, and fractured communication.
Traditional ERP and QMS systems often struggle to connect these disparate points. Manual reporting, batch reconciliation, and siloed data sources introduce delays and create blind spots. Without shared visibility across functions and partners, the risk of failure increases.
True visibility means detecting issues early, tracing them to their origin, and modeling solutions in real time. It empowers teams to act on dynamic, interconnected data – not anecdotal updates or stale reports.
It’s not about dashboards for their own sake, it’s about actionable intelligence that links strategy to execution and frontline operations to leadership decisions.
Where Visibility Breaks Down
The middle layers of the supply chain; Tier-2 and Tier-3 suppliers, regional warehouses, and logistics handoffs are the most common points of failure. These gaps arise from tribal knowledge, outdated systems, and fractured communication rather than negligence.
Legacy ERP or QMS systems often struggle to connect these disparate nodes. Manual reporting, batch reconciliation, and siloed data create delays and blind spots. Without shared visibility across functions and partners, the chances of surprise – and failure – increase.
That’s where integrated systems like our Integrated Pharmaceutical Management (iPM) platform play a crucial role. iPM is designed to connect pharma operations with traceability and intelligence across the entire value chain, from sourcing to delivery, without overcomplicating compliance or operations.
Visibility and the Stakes in Pharma
Pharmaceutical supply chains are unlike any other. Cold chain handling, lot-level serialization, regional regulatory constraints – these aren’t edge cases, they’re daily realities. Each handoff is a potential liability, and each process deviation can result in safety concerns, product loss, or noncompliance.
Visibility enables faster detection of temperature excursions, more accurate documentation of chain-of-custody, and tighter control over manufacturing variances. It allows companies to react to disruptions – before those disruptions reach the patient.
Beyond risk, visibility is also a source of trust. Providers, regulators, and patients must have confidence in how drugs are sourced, stored, and delivered. Without reliable transparency, trust erodes – and with it, a competitive position.
From Compliance to Confidence
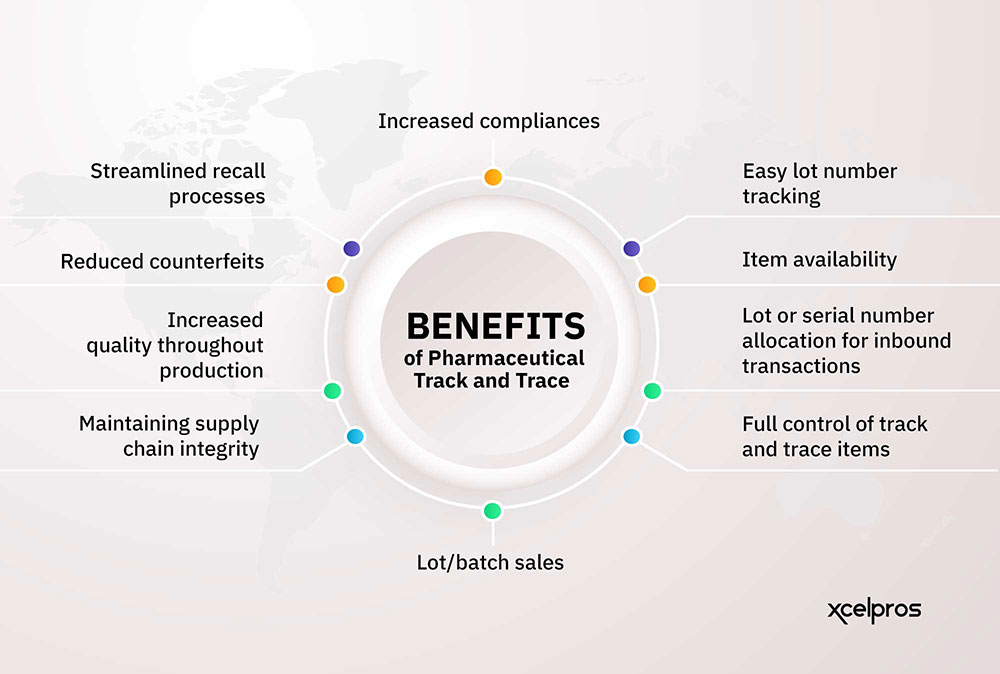
Visibility in pharma has long been tethered to compliance. Regulations like the Drug Supply Chain Security Act (DSCSA) and EU Falsified Medicines Directive (FMD) require traceability. But forward-thinking organizations are using that same visibility infrastructure to drive broader outcomes: more efficient inventory management, smarter production planning, and accelerated speed-to-market for new therapies.
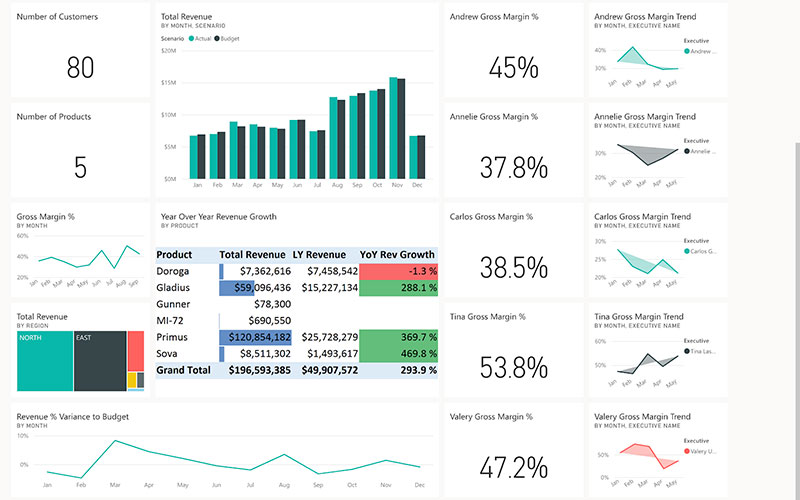
The shift from viewing visibility as a cost of compliance to a strategic enabler is underway. When executives gain real-time access to critical KPIs – from supplier performance to delivery timelines – they can shift from damage control to proactive planning.
What a Modern Visibility Strategy Looks Like
A modern visibility strategy is not a patchwork of tools, it’s a framework. It blends real-time analytics, machine learning models, and sensor-driven monitoring with process discipline and cross-functional alignment.
The foundation is clean, connected data. That means:
- Consolidating systems that track production, logistics, quality, and finance.
- Embedding smart tags (like RFID or QR codes) to enhance tracking accuracy.
- Using AI to detect anomalies in delivery routes, batch trends, or demand spikes.
- Establishing governance structures that make insights actionable across departments.
This is how visibility becomes more than just a mirror – it becomes a mechanism for agility, resilience, and decision velocity.
Why This Matters Now
2025 is not a year of stability. Global distribution remains volatile. Regulations are evolving. Workforce turnover is affecting tribal knowledge and process continuity.
Meanwhile, pharmaceutical companies are being asked to deliver more – faster, cheaper, and safer. Visibility is the only way to meet those expectations with confidence. It allows companies to manage the present while preparing for what’s next: whether that’s scaling production for a new drug launch or responding to a new serialization mandate.
Without visibility, every decision becomes guesswork. With it, companies unlock a measurable advantage.
Closing the Gaps
The journey toward full supply chain visibility doesn’t begin with a platform – it begins with alignment. Visibility fails not because tools are missing, but because teams are misaligned, processes are fragmented, or data isn’t trusted.
That’s why the first step is to identify the blind spots. Map where data lives. Document where delays tend to occur. Understand where tribal knowledge still governs critical workflows. From there, invest in systems that unify, not just automate.
And remember: visibility isn’t something you “implement.” It’s something you build into the foundation of how your organization operates.
Conclusion
Visibility isn’t a box to check. For pharmaceutical companies, it’s the bridge between today’s complexity and tomorrow’s confidence. It ensures compliance, protects patients, and empowers your teams to operate at the speed modern pharma demands.
If your organization is evaluating how to improve visibility across its supply chain, or struggling with fragmented operations – it may be time to rethink your approach.
Let’s map a better strategy, together. One that moves beyond checklists and dashboards – and into smarter, connected, pharmaceutical operations built for what’s next.
Set up a time to talk