Introduction
Every business leader has heard the term “sustainable manufacturing,” but not all know that practicing methods that help the environment can also grow their business.
“Sustainable manufacturing is the creation of manufactured products through economically-sound processes that minimize negative environmental impacts while conserving energy and natural resources,” the United States Environmental Protection Agency states. These same practices enhance employee, community and product safety in part by producing less waste that pollutes the air, water and soil.
According to the EPA, companies that use a methodical, planned approach to sustainable manufacturing processes:
- Increase operational efficiency by reducing costs and waste
- Respond to or reach new customers and increase competitive advantage
- Protect and strengthen brand and reputation and build public trust
- Build long-term business viability and success
- Respond to regulatory constraints and opportunities
Fostering Growth
These environmentally friendly sustainable manufacturing practices help companies grow by reducing production costs long term. For example, instead of paying thousands of dollars each month to an electric company to light and cool a 300,000 square-foot manufacturing plant, consider covering a flat roof with efficient solar panels.
The average payback time for a home solar electric installation (industrial estimates were not available) is roughly 6-10 years, though it varies depending on the climate and other factors. Solar panels also tend to last 25-40 years meaning roughly three-quarters of their useful lives is spent generating free electricity. The most recent designs are much more efficient, producing more power in a smaller size, than those made 10 years ago. The result is greater efficiency, allowing manufacturing facilities to cover less of their roofs while producing as much or more power than the older models.
Production plants can also reduce their massive electrical bills with skylights. The waterproof domed coverings help illuminate work areas, reducing the need of electric lighting. Extended exterior shelves can reduce sunlight, cutting cooling costs.
Figure: 1 Sustainable Manufacturing – a Big Picture
Turning Trash Into Treasure
Other sustainable methods look at ways to reduce waste, especially by converting some “trash” into new products or using it for new methods.
One website alone lists 35 artful ways homeowners can recycle wooden pallets. These new uses include making tables, bed frames, stairs, mounting frames for heavy electronic display monitors and a host of other uses. Many of these same methods work for industrial companies in terms of outfitting conference rooms and other non-work areas.
From an industrial perspective, worn pallets can be repaired, cleaned and reused. They can also be sold, recouping some of the cost. Other uses for worn pallets include chipping them, turning them into wood pellets. The pellets can then be burned, generating heat and electricity.
Get a consultation to discover more about building sustainable operations for your manufacturing company.
Cascading Chemicals
Recycling is a large part of the sustainable “green” economy. Industrial chemicals can be recycled. They can also be reused through a process known as “industrial symbiosis,” greenbiz.com states. One example cited uses ferric chloride, which is a byproduct of steel pickling in hydrochloric acid, to treat water.
“Frequently, recycled chemicals are not only cheaper than newly produced ones, but they also reduce resource consumption, waste generation and greenhouse gas emissions. The carbon emissions through solvent recycling are 46 percent – 92 percent lower than those of new solvent production,” the website states.
When the article was written in 2019, industrial giants Siemens and Evonik were conducting research to convert the most common greenhouse gas—carbon dioxide (CO2)—into common industrial chemicals such as ethylene.
Other methods used to reduce chemical and industrial waste cited by greenbiz include swapping what might be one manufacturer’s trash with a different nearby business. That business can use these materials in its products.
Another environmentally friendly industrial method is “leasing” chemicals. In this model, a manufacturer sells the functions performed by the chemical using functional units, not the chemicals themselves.
Large manufacturers with their own wastewater treatment plants can redesign those facilities in ways that help the company turn a profit and grow. Companies interested in practicing sustainable manufacturing practices can modify existing equipment to produce energy, clean water and chemicals because, “the future of sewage is power and profits.”
The greenbiz.com article ends with a quote made in 1848 by the former president of the London Royal College of Chemistry, R.W. Hoffmann: “In an ideal chemical factory there is, strictly speaking, no waste but only products. The better a real factory makes use of its waste, the closer it gets to its ideal, the bigger is the profit.”
Technology Can Spot Opportunities
One way a company can practice sustainable operations management is by using its data wisely. Especially in forward-thinking firms that use internet of things (IoT)-enabled devices, they have access to mountains of information.
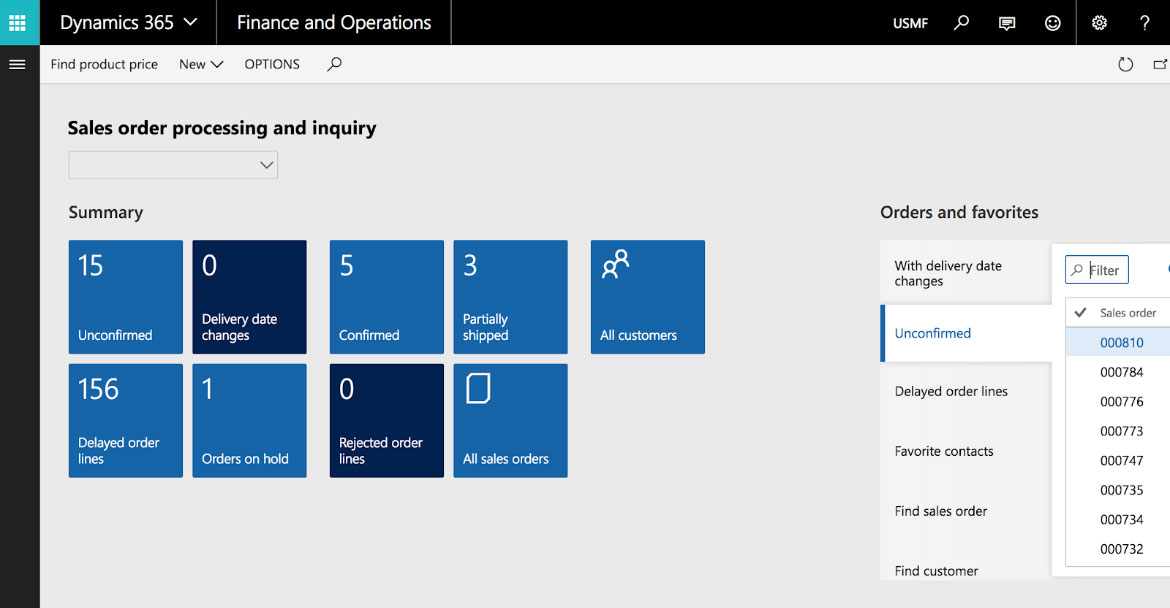
Combining a well-thought plan with the right software lets these firms look at everything coming into their warehouse—including packaging—as potential profit sources. Enterprise resource planning (ERP) products such as Microsoft Dynamics 365 and its Supply Chain Management Module let companies of any size keep accurate track of their inventories. Add in the Integrated Chemical Management component and chemical manufacturers have an accurate label management solution that also produces safety data sheets.
By understanding the chemicals involved and working with sustainability experts, plant managers can evaluate their current conditions.
Executives interested in sustainable production and consumption—and being more competitive—will want to ask questions similar to these: What current waste products and materials can we use for secondary purposes or repackage and sell to someone in a different industry? Can we reuse packing materials we receive to pad and protect outgoing shipments? Are we using our raw materials effectively or are there ways we can become more efficient? How much power do our plants use? Are there affordable ways of reducing that consumption while also generating some of our own power all while meeting our long-term business goals?
Asking questions like these, and then using powerful software to find the answers, help innovative firms generate more money. That in turn can use sustainable practices to fuel growth.
The Bottom Line
Sustainable manufacturing involves looking at everything a company has, from a different angle. More office employees are working from home, freeing up space. Can we use that space for a different purpose instead of looking at empty desks? Can we move items around and expand our production facilities or our warehouse without having to build or buy new facilities?
Operations managers wanting to fuel growth by reducing power consumption can use ERP software to find ways to save money and new ways to make money. All it takes is a little outside the box long-range thinking.