- There’s a consistent demand to scale capacity when it comes to pharma manufacturing as healthcare demand continues to rise globally.
- Cost-savings, shorter turnaround time, and enhanced productivity are some of the key criteria for top executives in pharma manufacturing companies.
- Automation of production lines, minimizing raw material wastage, stakeholder synchronization, optimized resource allocation, etc. are some of the ways with which pharma manufacturing can be streamlined.
Around the world, we continue to see rising demand for access to quality healthcare. Side-effects of the recent pandemic significantly added to this demand. This has placed a lot of pressure on pharma and biotech manufacturing companies, who are finding it difficult to:
- Streamline research and development (R&D) processes.
- Reduce overall costs and improve time to market.
- Ensure 100% safety and regulatory compliance.
- Enhance production capacity.
- Expand market reach.
Pharma manufacturers must constantly upgrade their game regarding R&D, operations, production, and distribution with newer technologies and strategic business moves. With signs indicating the industry is poised for extraordinary growth, it’s becoming a given that manufacturers will need to invest in leaner, more agile production processes.
According to the 2020-2027 Pharmaceutical Market Size Report, by Grand View Research, the global pharmaceutical manufacturing market size was valued at USD 324.42 billion in 2019 and is expected to grow at a compound annual growth rate (CAGR) of 13.74% from 2020 to 2027.
These growth stats put focus on the need for ramping up production without compromising on safety, all while ensuring consistent profitability.
Key Factors in Pharma Manufacturing
Before we look into how manufacturing in pharma companies can be streamlined, we must consider some key processes involved.
1.Robust R&D: For pharma and biotech companies, continued investment in laboratories is essential in ensuring long-term success. With Robust R&D comes increased chances for innovation, which can define a pharma company’s overall market position in terms of being the first to manufacture a ground-breaking formula. The strategic movement towards streamlined manufacturing begins with ensuring superior, quality research in the labs.
2.Raw Material Acquisition and Distribution: Whether it’s small-molecule or biological drugs, pharma companies typically depend on an intricate network of raw material manufacturers and distributors to acquire safe and superior-quality products. In addition, complex formulations require compounds manufactured across multiple facilities to be stored and transported in optimal conditions. Manufacturers could be dependent on multiple different suppliers for raw materials globally.
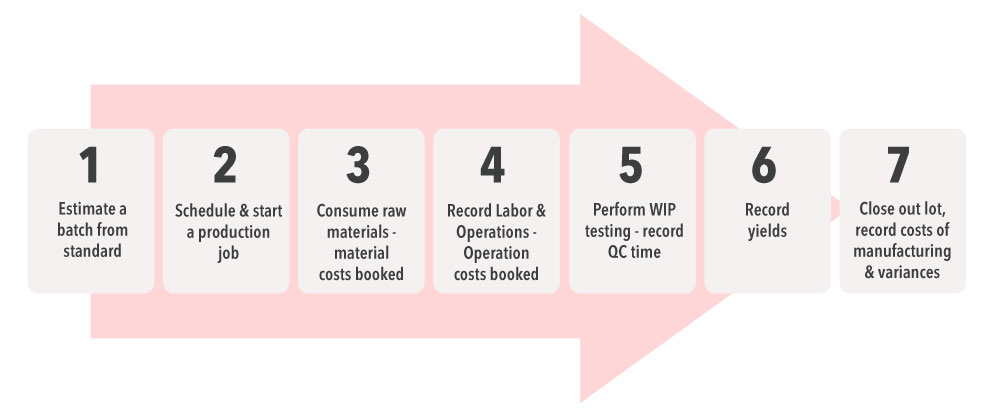
3.Managed Production Lines: Today, pharma companies are more dependent than ever on fast-paced production lines backed by technology-enabled batch manufacturing, serialization, and traceability. Bridging the gap between hardware and software for streamlined drug production can make a huge difference in speed to market.
| Manufacturing Process | Issue/ Roadblocks | How Technology Can Help |
|---|---|---|
| R&D | Prone to human error, slower processes | Automated data integration and analysis, AI for molecular identification |
| Supply Chain | Highly complex, data discrepancies or duplication, missing information, stock-outs | Centralized SCM for real-time visibility, centralized data access, real-time stakeholder communication, inventory management in ERP |
| Drug Manufacturing/ Production | Shop floor to top floor communication glitches, communication time-gaps, human errors in reporting/ record maintenance | Automated production lines, report generation in ERP, real-time communication between shop floor and top floor |
| Quality Control | Counterfeits, fake drugs, human errors, formulation errors | Computerized serialization, use of blockchain to ensure drug safety |
4.Competent Supply Chains: Healthcare is a global business and, now more than ever, pharma manufacturers are dealing with complex supply chains involving multiple stakeholders spread worldwide. Ensuring that these supply chains are competently managed is critical to ensuring the overall streamlining of pharma manufacturing.
5.Quality Checks: When it comes to drug manufacturing, anything less than 100 percent is often unacceptable. Pharma companies are well aware of the perils of lawsuits, license cancellations, and other dire consequences regarding quality management. At every stage of pharma manufacturing, quality checks are paramount to ensuring drug safety and compliance with all required healthcare regulations.
Schedule a call to learn more about how to streamline your pharmaceutical manufacturing production process
How Can Pharma Manufacturers Streamline Their Production Processes?
Pharmaceutical companies are embracing newer and newer technologies for quicker results, better process management, and enhanced productivity. Still, there’s a lot more that pharma manufacturers and their CDMOs can do to enhance the overall pharmaceutical production process for significantly better results.
- Integrating new technology in the lab is proven effective in accelerating research and innovation. Leveraging Big Data and Analytics for data collation, integration, and insights generation from clinical trials can expedite the process and ensure accuracy and transparency. Similarly, computational permutations are effective in molecule identification for a particular drug. Gene sequencing, digital record maintenance, computerized medical equipment, etc., are becoming game changers in strengthening R&D and the production process typical in pharma manufacturing.
- Pharmaceutical shop floors can and should be well-integrated with the IT infrastructure on the top floor. By embracing software-managed production lines, manufacturers can leverage automation for faster and error-free processes. Similarly, production supervisors can benefit from the automated data flow from an Enterprise Resource Planning (ERP) system to and back from the production lines. This software can manage tasks like reporting and serialization to save time and cost.
- Newer technologies and software for SCM are becoming pivotal in helping pharma companies stay on top of complex supply chains and distribution networks. IoT solutions are leveraged in pharma manufacturing and distribution for real-time monitoring and communication. Better shipping times and inventory management become possible through effective data analysis. Many companies also use blockchain to ensure data security and encryption while managing complex supply chain networks globally.
- The use of blockchain and comprehensive ERP software (for serialization) are also helping manufacturers ensure drug safety. Since these tools and technologies provide the option of complete traceability (from production to patent), drug counterfeits become extremely difficult, if not impossible. Drug quality and safety are major concerns for manufacturers, and optimal use of technology can ensure quality checks, thereby saving efforts, costs, and time.
Final Thoughts
Pharmaceutical manufacturers are embracing newer technologies for better production and profitability. With these technological advancements, companies could achieve their manufacturing goals without compromising quality and safety.
- Newer technologies and software such as IoT, Artificial Intelligence, Data Analytics, SCM, and ERP play important roles in streamlining manufacturing processes in pharma.
- Pharma companies need to reinvent themselves technologically to keep up with the complex and ever-expanding canvas of global healthcare.
Resources: Workflow Software for Improved Healthcare Solutions