Why Pharmaceutical Manufacturers Should Choose Microsoft Dynamics 365 Finance and Supply Chain
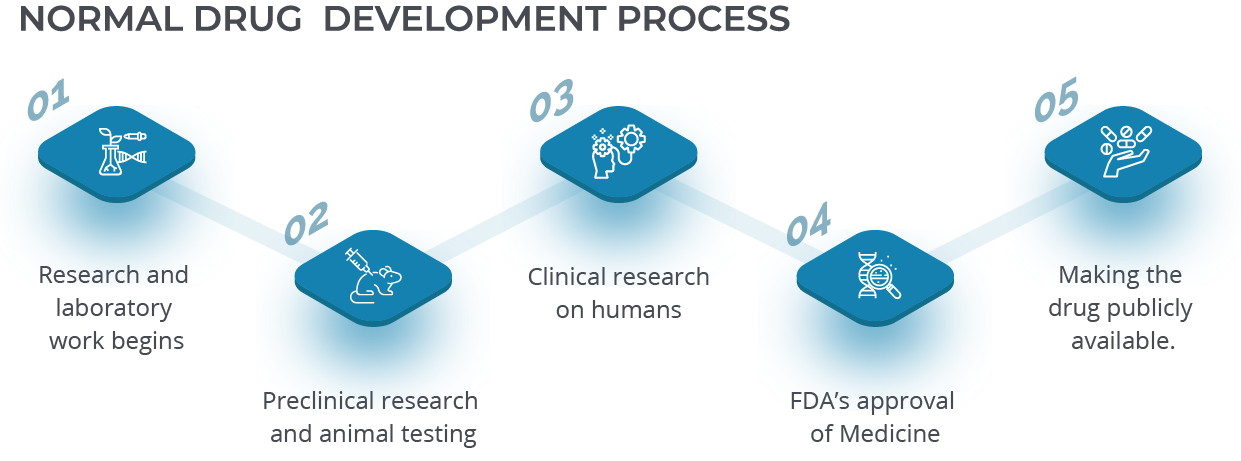
In the pharmaceutical industry, there’s a constant need to manage different challenges such as ever-changing regulations, severe production environment difficulties and complex equipment that can be difficult to maintain. As the pharmaceutical industry continues to evolve and grow, understanding and adopting intelligent technologies like Dynamics 365 becomes more and more apparent. For more information see the full article here.
Get started with one week no-obligation pilot to experience Microsoft Dynamics Finance & Supply for Pharmaceutical