Introduction
As the world continues to deal with one of the deadliest pandemics in modern history, governments are working overtime to protect their citizens from the deadly disease known as Covid-19.
With the potential impact of Covid-19 on pharmaceutical sales estimated to be huge, two types of drugs are being sought after:
- Vaccines to keep humans from catching the disease
- Therapies to treat people with the disease and help them recover
At the end of 2020, the U.S. Food and Drug Administration (FDA) modified its approval process for vaccines, issuing an emergency use authorization (EUA) to Pfizer-BioNTech for a vaccine to prevent coronavirus disease 2019 (COVID-19). The initial EUA applied to vaccines for people 16 and older. It was further modified on May 10, 2021 to include adolescents ages 12-15. The EUA lets the vaccine be distributed in the US. Similar documents were issued to Moderna, Inc. and Johnson & Johnson / Janssen. On July 9, 2021, Pfizer said it would seek approval for a booster shot to target the newer variants of the disease. The FDA and other regulators have, at this time, disagreed with the need for it. This could result in Pfizer share price climbing by as much as 66% in 2021, as suggested by analysts from investors.com
Companies might want to rethink their pharmaceutical product launch strategies. This change in the FDA approval process may require changes in pharmaceutical new product launch plans, prioritizing Covid-19 treatments over other medicines. These plans affect not only products sold in the U.S. but also in the Indian pharma market with its 1.4 billion residents (four times that of the U.S.).
Using enterprise resource planning (ERP) software can help pharmaceutical companies gain regulatory approval of their drugs and treatment plans.
By the Numbers
Expected 2021 sales from Covid-19 vaccine makers:
- $15 billion-$30 billion: Pfizer/BioNTech (share price +1.8% for Pfizer, +156% for BioNTech)
- $18 billion – $20 billion: Moderna (share price +372%)
- $10 billion: Johnson & Johnson (share price +7.7%)
Five other companies are also making Covid-19 vaccines but none have been approved by the FDA yet. (Source: The Guardian)
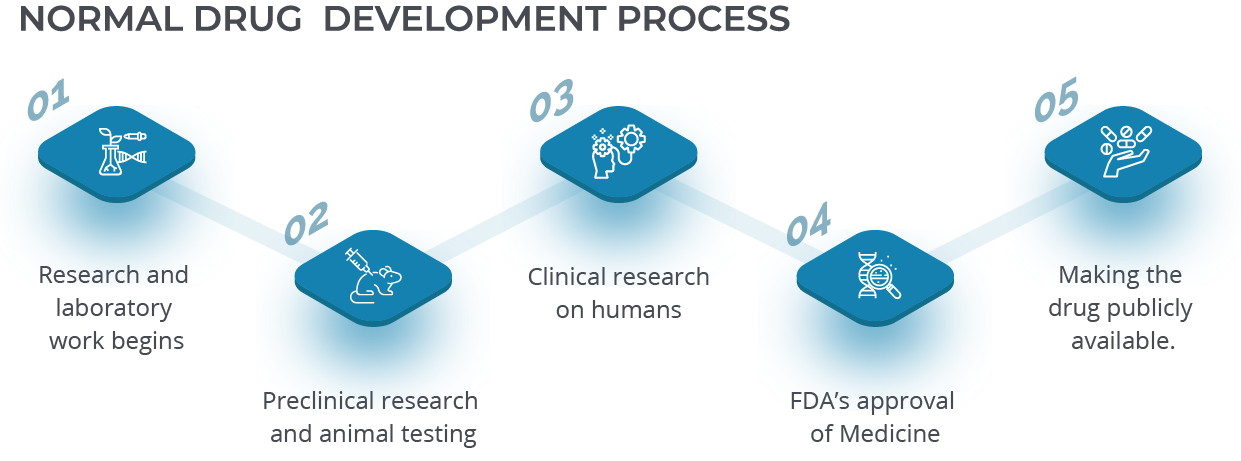
Normal Drug Development Process
The normal pharma go to market strategy requires a clear long-term view since most medications take 10-12 years to go from the laboratory to the medicine cabinet. Full FDA approval requires six months of data plus another six months for review before official approval is given. These additional steps then come at the end of the drug creation journey:
- 1.Research and laboratory work begins.
- 2.Preclinical research and animal testing looks into the drug’s safety for human beings.
- 3.Clinical research begins on humans, typically comparing test results from patients getting the therapy to those receiving a placebo.
- 4.The FDA reviews the data and then decides to approve or disallow the medicine.
- 5.The FDA monitors the drug for safety once it becomes publicly available.
The Covid-19 vaccines are examples of drugs required to combat a crisis, one that has already killed more than 606,000 U.S. citizens and 4 million people worldwide. They present different pharmaceutical marketing challenges than existing medications.
“An EUA can be given if there are no adequate or approved alternatives,” WKYC of Cleveland, Ohio states. Pharmaceutical manufacturing companies still need to prove the drug is safe by thoroughly testing against thousands of study participants.
“The only difference really between the emergency use and the licensure is that volunteers are observed for a longer period of time to see the duration of protection and if there might be rare adverse events that occurred down the road,” WKYC quotes Dr. William Schaffner of Vanderbilt University as saying.
Figure:
Difference Between EUA and Standard Approval
Drugs with full FDA approval have several major advantages over those with just an EUA, including:
- The medications stay on the market after the pandemic is no longer an emergency
- EUA-approved therapies must be pulled from the market
- Medicines still in the development pipeline may be tested against newer, more drug-resistant, variants
- The pharmaceutical manufacturer can market directly to consumers
- After full approval, businesses can require all employees to be vaccinated, WKYC states
The FDA Requires Records
According to the FDA’s Code of Federal Regulations (CFR), Section 312.57
“Recordkeeping and Retention,” a drug sponsor (i.e., manufacturer), “shall maintain adequate records showing the receipt, shipment or other disposition of the investigational drug. These records are required to include, as appropriate, the name of the investigator to whom the drug is shipped and the date, quantity and batch or code mark of each shipment.”
Records must be kept for two years after the marketing application is approved or for two years after shipment and delivery of the drug for investigational use is discontinued and the FDA notified.
Traditional Recordkeeping is Cumbersome
Many pharmaceutical companies still use spreadsheets to keep track of records. However, they often get data from a single source or department. Multiple sources may mean mixed-up or missing records, slowing the approval process.
Typically, companies using older software tend to silo their data. Inventory has its records. Finance has its own. Sales and marketing have theirs.
The problem in terms of regulatory compliance is that none of this information is shared across departments.
Enterprise resource planning software (ERP) such as Microsoft Dynamics 365 Finance and Operations lets pharmaceutical manufacturers gather information from all of these different sources. The data is combined into one unified whole.
Dynamics 365 can then automatically generate labels. It allows companies to track everything from large batches to individual doses, making FDA compliance simple and easy.
Data comes into the ERP network from sources scattered literally all over the globe. Real-time information is available with the click of a mouse or typing a few keystrokes.
Dynamics 365 data is securely stored on Microsoft Cloud servers. It’s available any time, anywhere. Executives can obtain any record in the system quickly and easily, ensuring compliance with FDA regulations. This lets executives provide accurate data to regulators quickly and easily.
Summary
Covid-19’s death toll led the FDA to accelerate its approval process from 10-12 years to a mere matter of months. Moving forward, agile pharma go to market strategies that can adapt to these changing requirements will undoubtedly be more profitable. An effective ERP software solution such as Dynamics 365 helps pharma companies adapt, and be able to quickly provide any required regulatory documents in order to remain compliant.
Get a consultation on pharmaceutical go to market strategy during challenging times.





