Introduction
The business world has changed dramatically with the rise of digital commerce, and companies have had to rethink their strategies and business models to accommodate it. What was once just an idea in science fiction has become reality, and the phenomenon’s significance cannot be overstated. Data-driven technology such as artificial intelligence (AI) and machine learning are at the heart of this change, quickly redefining how people buy goods and services—and companies operate. Microsoft Dynamics 365 has been at the forefront of supporting these shifts, but what does that mean for businesses?
Digital commerce reaches all industries
Before we get into how digital commerce has grown after COVID-19, it is important to recognize that it is not only retail and service industries where ecommerce channels are on the rise. For example, consider chemical and pharmaceutical firms. Many are using ecommerce channels to market and sell branded products, operate online stores, or promote product recall information. These companies benefit by limiting overhead costs like warehouse storage space, labor for packing and shipping goods, etc. And consumers benefit from convenience: shopping is available at any time on one website instead of having to navigate different websites for a particular product.
In addition to their brands’ products, manufacturing firms have taken advantage of grey marketing opportunities by selling private labels via sites like Amazon’s marketplace or Walmart’s marketplace seller platform. They can use these channels to capitalize on popular products before they’re out of stock or completely sold out and use them as a bridge to launch new brands.
While ecommerce channels were primarily used to sell direct competitors’ products, now many manufacturers can also sell their own branded products through these platforms. The manufacturer then takes care of logistics and fulfillment while taking advantage of the branding expertise required for success on these media types.
Regarding compliance and regulation, manufacturers must keep up with complex regulatory requirements like FDA 21 CFR Part 11 rules when designing an electronic record-keeping system. Part 11 mandates among other things rigorous change control procedures and audit trails to track data modification which may be difficult in dynamic environments.
These are all important reasons why you need a powerful solution for managing your supply chain management (SCM) processes – including business process automation tools such as Requisition Approval Management (RAM), Order Approval Management (OAM), and Warehouse Execution System (WES).
eCommerce after Covid
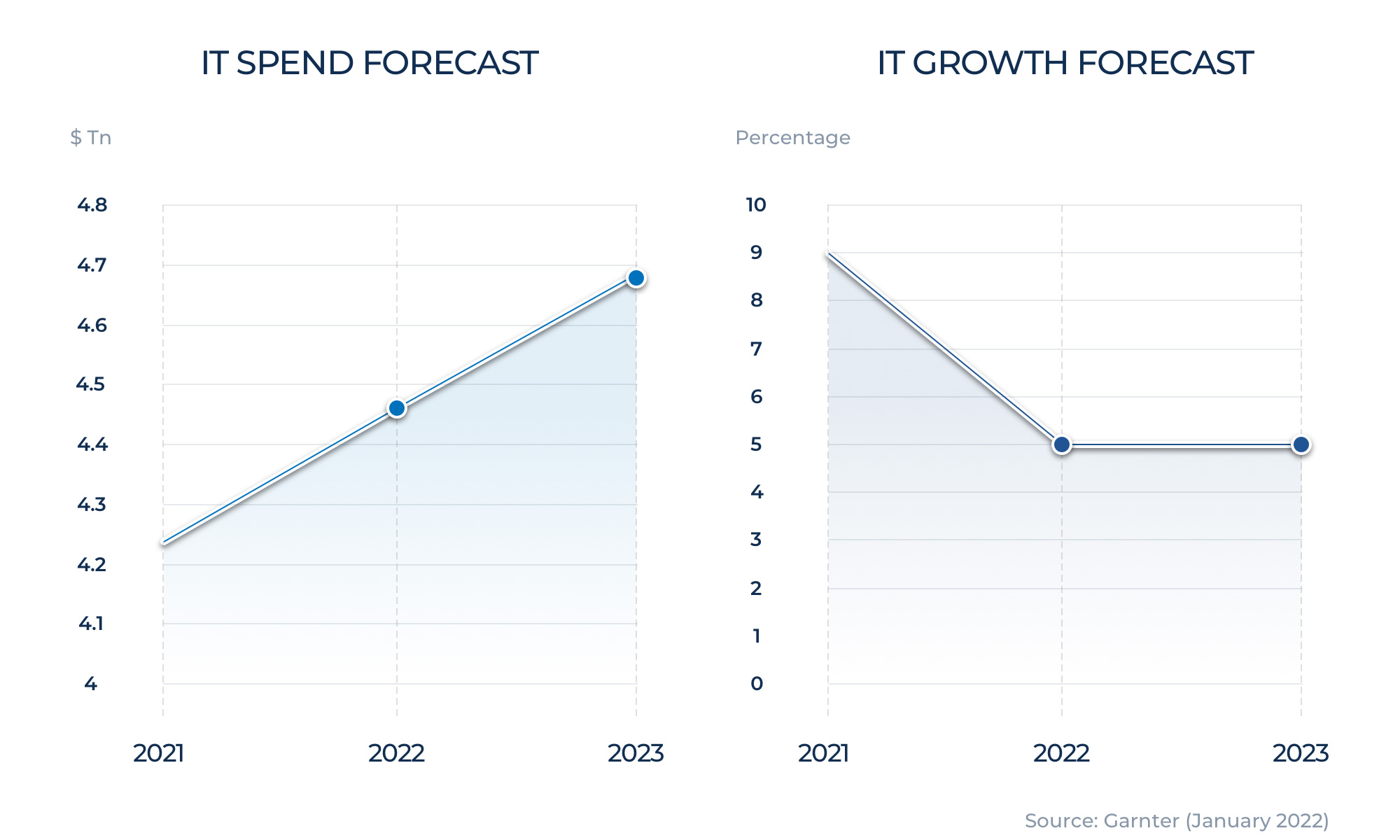
$571.2 billion in 2019 to $815.4 billion in 2020. An increase of $244.2 billion, or 43%.
Figure 1:Growth of eCommerce after Covid-19

Source: census.gov
It’s hard to believe that it has been just over a year since COVID-19 rocked companies and their business strategies the world over. With another year quickly coming to an end, it’s a relief to see more companies have been able to move on from recovery and start rebuilding and reinventing their digital commerce strategy. But there are key considerations for regulated industries like pharmaceuticals and chemicals.
COVID-19 came at a pivotal time for regulated industries with compliance obligations for data protection laws and supply chain requirements. Before the pandemic, executives were just starting to wake up to these regulatory trends with increasingly complex compliance mandates; but after Covid, many took note that this wasn’t a problem that would go away by itself. Industry players realized they needed to be ready for change.
This global event drove a significant change in eCommerce strategy for many industries, including B2C sales and B2B business-to-business interactions where some organizations’ data security practices may have been less diligent.
As businesses struggle with ongoing supply chain and staffing issues, many have been forced to look more closely at new ways to deliver their goods to customers faster, more reliably, and with increased safety from outside threats. The evolution of courier services has helped lead to changes in logistics processes – assisting regulators in maintaining visibility into product movement while enabling enterprises to protect themselves against potential attacks.
Another major shift has been focusing more on customer experience rather than simply looking at what can be done internally. Companies should now recognize that it’s not only about providing an effective process or product, but now more than ever, it’s also about delighting the customer – a point that’s become incredibly important.
These factors are just a few driving the need for an up-to-date solution that helps achieve both goals by ensuring transparency and traceability across every communication channel through integration with platforms like Microsoft Office 365 so your enterprise can reduce complexity without sacrificing efficacy or control.
For companies in highly regulated industries like pharmaceuticals and chemicals, it’s easy to recommend Microsoft Dynamics 365, designed specifically for global, large multinational enterprises operating in highly regulated environments who need real-time access to consolidated company data across multiple divisions around the world.
Microsoft Dynamics 365 and eCommerce
It’s no secret that eCommerce has been growing steadily for years. From big players like Amazon to newer start-ups, digital commerce is poised to make even more inroads into all corners of our economy. The last few years have also seen a dramatic change as retailers realize that online and mobile channels are needed to stay competitive. Of course, all this begs the question: what role does Microsoft Dynamics 365 play here? What can it do to support these new needs?
Microsoft is committed to helping organizations overcome today’s business challenges through the continued development of innovative solutions and services. The award-winning Dynamics 365 platform provides customers with powerful tools to drive success across their entire organization – empowering employees to do more daily with mobile access anytime and anywhere.
Overall, Dynamics 365 enables businesses agility, flexibility, and speed when developing new capabilities or meeting evolving customer demands. In addition, Dynamics 365 Ecommerce features can offer an out-of-the-box solution for any business—regardless of size or industry—to sell their products online with robust features and functionality.
With Dynamics 365, customers get access to powerful web store templates so they can create unique shopping experiences that resonate with their customers. These include customizable pages and easy-to-manage checkout options for buyers. And of course, Dynamics 365 includes advanced payment processing integration, so retailers have seamless access to existing payment providers without having to spend time building and maintaining these integrations themselves. So, while global economic developments will undoubtedly impact how digital commerce continues to evolve in different industries over time, Microsoft Dynamics 365 is well positioned today as a flexible platform that meets the needs of companies across regulated sectors who want to leverage this burgeoning channel without sacrificing security or compliance.
Beyond support for customer service operations, Microsoft Dynamics 365 provides robust enterprise resource planning (ERP) capabilities – including finance, accounting, inventory management, and production – which can make it easier for leaders within regulated industries to maintain compliance within the evolving landscape of privacy regulations without making compromises on productivity or efficiency.
As regulations continue to evolve, many institutions will find themselves reviewing their vendor ecosystems and assessing whether there are adequate controls in place should they be subject to regulatory scrutiny. Companies should take care to review how specific vendors’ offerings align with their current compliance requirements including data governance, confidentiality and protection of personal information (COPPI), anti-money laundering (AML) or know your customer (KYC) requirements.
Next steps
So, what are the next steps for businesses looking to utilize e-commerce channels?
It’s important to do some research – companies should research the types of technology that can be implemented to drive digital commerce. With any new change comes many opportunities and risks that must be carefully considered.
Talk to your partner business software providers You should consider the type of retailing experience you want customers to have by speaking with the IT vendors that serve those areas. Microsoft Dynamics 365 is a powerful business solution and partners with many leading companies in areas such as forecasting, supply chain management, order fulfillment, human resources, financials and more.
The continued success of this solution stems from its robust integration capabilities. As a Microsoft Partner, our clients don’t want to go through the process of switching between multiple systems; they wish to have one system to handle it all.
Schedule a call now to see how we can support your company’s growth.