Introduction
Pressing on after the height of the pandemic, the manufacturing industry continues to struggle with workforce and supply chain challenges at every level, including increased costs for both physical goods and employee wages.
While we continue to be hopeful for significant growth in U.S. manufacturing, for many, optimizing their manufacturing processes has been vital to improving their bottom line and productivity and getting more out of their operations, helping them remain more profitable overall.
Working with the right partner can make manufacturing easier in many ways, whether it’s lower costs or increased productivity. A partner that understands manufacturing software and understands your business is most likely going to be able to deliver solutions that fit both your present needs and your future goals.
Technology and Modern Manufacturing
Technological advances have always been a boon to manufacturing businesses, helping them to increase efficiency and optimize even the most minor processes.
With rapid changes in consumer demand and the rise of globalization, companies need an integrated enterprise system that helps them manage their data more efficiently and productively. With a robust ERP solution and reliable hosting infrastructure, we’ve helped companies in industries like automotive, paper processing, consumer goods, textiles, distribution centers, and oil & gas manage global growth.
Today, most manufacturers choose Microsoft Dynamics to streamline their operations further.
Microsoft Dynamics is built on cloud architecture, which means you can access the same tools and resources on any device, no matter where you are or your company. And because Microsoft Dynamics is built on Azure – one of the world’s most significant clouds – customers get access to Azure’s security features, including comprehensive firewall protection, DDOS protection, and secure data transfers so critical information stays safe from cyber-attacks.
This flexibility is essential for manufacturing industries because it allows them to work anywhere in the world without worrying about backups or even localized power disruptions. That said, businesses should also consider offline capabilities when selecting a cloud ERP vendor, as they will help mitigate downtime while ensuring uninterrupted production flow during natural disasters or other emergencies.
Reasons For a Technology Partner
If you’ve been a manufacturer in business for even a few years, it’s fair to say that you know having a partner to help you optimize your processes can be the difference between success and failure.
By working with the right partner who understands your business needs and software like Microsoft Dynamics 365, you can ensure that you’re getting the most out of your technology.
Here are seven reasons why working with a partner who understands manufacturing and has experience with Dynamics 365 is so important.
- 1.Dynamic process optimization – It’s impossible for one person or team to optimize every process in their company. However, by collaborating with experts who understand how Dynamics 365 can integrate with other systems like transportation and compliance management, they can find ways to streamline business operations while cutting costs and increasing efficiency.
- 2.Better visibility – Some companies keep their inventory data in spreadsheets instead of a centralized system like Dynamics 365 which makes it difficult for managers to see where all the parts are coming from and going to. With the right kind of partners though, these manufacturers gain better visibility into their inventory levels which helps them avoid costly mistakes.
- 3.Outsourcing capabilities – Manufacturers often outsource work because there simply aren’t enough skilled workers available to do the job themselves. By outsourcing with a Microsoft certified partner, they can save money on labor expenses while ensuring quality control standards are met.
- 4.Streamlined processes – No matter what industry you work in, complex operations require expertise and knowledge outside of just one person or department. An expert will be able to map out an entire workflow more efficiently than someone without any experience at all.
- 5.Training & development opportunities – Manufacturers often have trouble finding time to invest in training because there always seems to be too much work on their plate already. A qualified partner will provide access to trainers who specialize in Dynamics 365, allowing employees to develop skills that benefit both the company and individual employees.
- 6.Saves time – Time is arguably one of the most valuable resources we have as humans. Working with a partner who knows what they’re doing saves everyone time and effort since it won’t take as long to figure out new software or new problems when they arise.
- 7.Builds relationships – The nature of manufacturing requires tight partnerships between manufacturers, distributors, suppliers, contractors, etc. One example is cross-training employees across multiple departments to create fewer bottlenecks and increase efficiency throughout the production process.
The Case for Microsoft Dynamics 365
If your main goal is optimizing your manufacturing processes, it’s important to utilize software that works for your business.
Figure 1:Technology of Industry 4.0
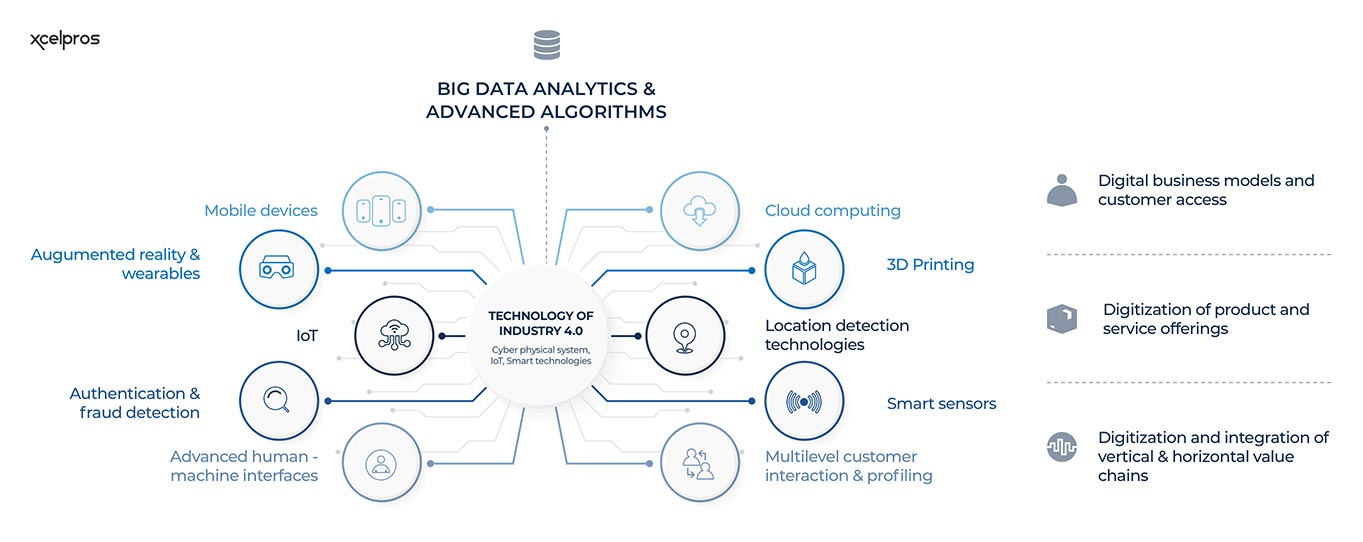
The world is changing and so is manufacturing. Industry 4.0, also known as the Fourth Industrial Revolution, is transforming the way we live, work, and communicate. It’s characterized by several new technologies and trends, including big data and analytics, cloud computing, IoT, artificial intelligence (AI), and 3D printing. Microsoft Dynamics 365 can help prepare your company for Industry 4.0 by providing features that support these new technologies and trends.
For example, Dynamics 365 can help you collect and analyze data from your manufacturing processes using IoT sensors, use AI to automate tasks and make predictions, and take advantage of cloud computing to improve scalability and flexibility.
In our experience, the best way to Optimize manufacturing processes has always been with Microsoft Dynamics 365. The reasons why more manufacturers are turning to Microsoft ERP solutions are simple: They’re designed from the ground up to streamline production and minimize errors – saving time and money in the long run.
Microsoft Dynamics 365 helps streamline several manufacturing processes:
- 1.Workflows – These automated tasks make day-to-day operations more efficient. You don’t have to remember everything since our system takes care of it.
- 2.CRM – Customer relationship management helps sales reps stay on top of their customer data, contacts, leads and follow-ups. All customer info is stored securely in one place instead of scattered across emails or office documents, which makes customer interactions more manageable than ever before. And if they’re not contacted within 24 hours after submitting a lead form, an email reminder is automatically sent to them.
- 3.Inventory – Keeping track of inventory items has never been easier. Just upload your item list and watch as they move through each stage of the production process until they are ready for shipping to customers. And if an item runs low on stock, an automatic notification lets you know when its reorder point has been reached.
- 4.Automation – Microsoft Dynamics 365 allows you to take advantage of pre-built integrations that save you time and effort. You can export sales orders directly into your ERP, automatically triggering production or assembly lines or scheduling work on manufacturing machines. When products are finished, send them to the appropriate warehouse and receive notifications when they arrive. You can also set up alerts to notify key personnel whenever an order arrives at any point during production.
No matter what type of manufacturer you are, there’s a Microsoft Dynamics 365 solution available for you. At Xcelpros, we consider the types of manufacturing processes you use and the size of your company before recommending the best solution. We’ll work closely with you so that we can be sure to understand all your needs before implementing a solution.
Additional Considerations
When working with partners to optimize your manufacturing processes, there are a few things to keep in mind. In addition to software considerations, like licensing and security, you’ll also want to consider things like reporting, data governance, and compliance.
Here are a few tips to help you get started
- 1.Determine if you need more than just business process automation – this is where Microsoft Dynamics 365 can be used for other use cases such as supply chain optimization or digitizing field services.
- 2.Understand that any IT project takes time, so start planning early by partnering with a company that has experience working with manufacturers and knows how to consider your specific needs.
- 3.Consider if what is being offered will integrate well into the business processes you currently have in place or if it will require additional resources for training or customizations to make it work seamlessly within your existing systems.
Next Steps
There may seem like a lot of things to consider when you start optimizing your manufacturing processes but taking the time to understand the challenges can make all the difference to the future of your business. While Microsoft Dynamics 365 is a powerful tool that can help you streamline your operations – it’s often more about working with a partner who understands your industry and unique requirements.
Do research, ask peers for referrals, and know what you want. With the right partner, you can optimize your manufacturing processes and take your business to the next level.
Contact us today to learn more!
References: What are Industry 4.0, the Fourth Industrial Revolution, and 4IR?






















