At a Glance
- Pharmaceutical manufacturing companies are investing more money and resources to assure adherence to regulatory compliance.
- Non-compliances need to be managed and tracked through their lifecycle, and using a digital platform eases the end-to-end process and follows it to completion.
- The first step towards regulatory adherence is to thoroughly understand the compliance requirements and form a dedicated process to comply.
Many pharmaceutical companies have restructured their financial and resource allocation models to invest more in adhering to compliance. Compliance requirements around the globe have grown in past decades. Each country has differences in safety standards, and global companies have to ensure they meet local requirements. Companies need better management and tracking of non-compliance. This article discusses the ways to reduce risks of non-compliance in the pharmaceutical industry. Manufacturers and distributors have dealt with compliance issues in the pharmaceutical industry. Companies have to conform to multiple complex and varying regulatory norms and safety standards. All the stages involve detailed compliance requirements, from procuring raw material to producing finished goods and quality testing of the final product. These complexities have further multiplied in recent years because of:
- Addition of several more regulations globally in the pharmaceutical and life sciences sector.
- Differences in the rules and regulations related to pharmaceutical compliance across different geographical regions.
- Absence of a viable infrastructure to manage and track non-compliance.
- Unclear SOPs and redundant record maintenance practices often lead to pharmaceutical manufacturing non-compliance.
Apart from these, non-compliance can result from other various smaller factors like faulty equipment, maintenance issues, faulty formula controls/ lab controls, etc. All contribute to quality compliance in the pharmaceutical industry. This is the reason that pharmaceutical and life sciences companies spend a fortune to avoid non-compliance. The costs of non-conformity are very high and thus, companies want to make sure that they adhere to the rules and regulations.
40%
of the pharmaceutical IT budget is spent on regulatory compliance.
Source: Gartner
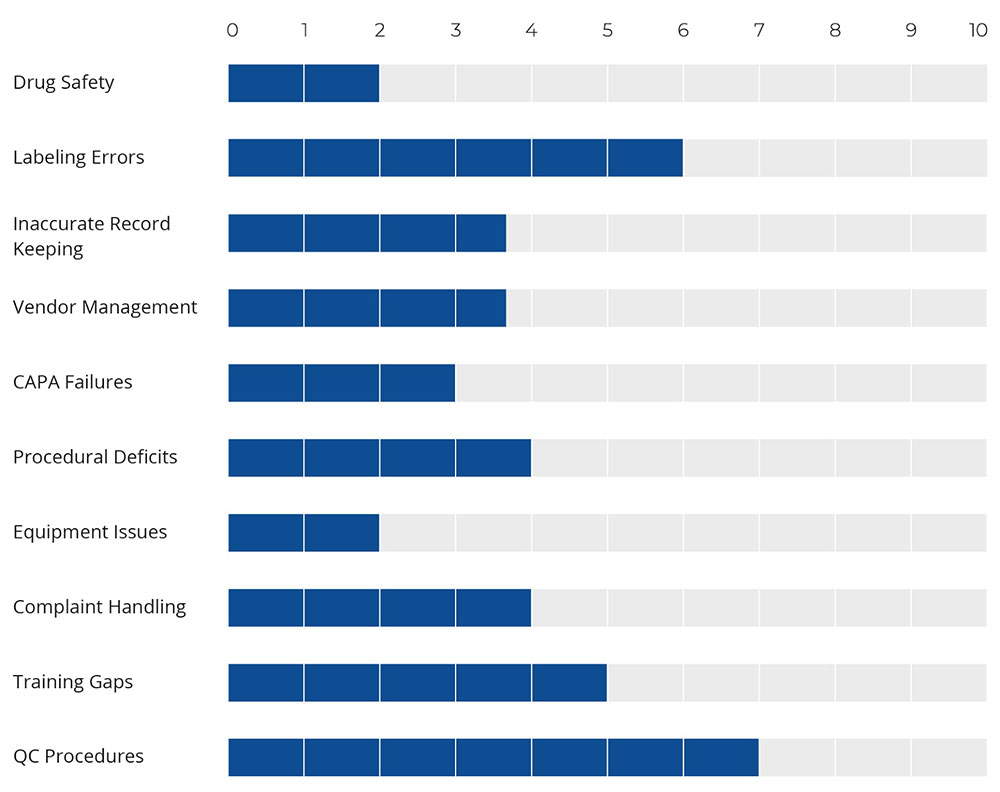
Figure: 1 Major Factors Affecting Compliance in Pharma
So how can pharma companies leverage technology to mitigate the consequences of non-compliance? Here are some ways that experts believe newer tools and innovations can help in better pharmaceutical compliance management:
1.Making use of the right tools: Documentation and record maintenance are a big part of the pharmaceutical industry’s compliance processes. Conventionally these documents have been manually maintained, which can lead to both errors and oversights. However, newer pharmaceutical software platforms come with the ability to gather and store data efficiently. It is essential to track user behavior and user audits to ensure compliance. A compliance management system with audit tracking and reporting tools can avoid non-compliance and improve overall product quality.
2.Leveraging integrated labeling: Pharmaceutical companies struggle to manage compliance as they enter newer territories and markets. There are different labeling practices and regulations which are challenging to manage. The labeling practices keep going through changes and updates even in familiar territories, making it essential for pharmaceutical companies to stay on top of these changes. To tackle labeling issues, companies can leverage the automated labeling platforms wherein the data can be auto defaulted from the different processes such as receiving, production and shipments. Inbuilt label printing within business process workflows avoids user errors and enforces process compliance. With integrated labeling within the ERP, users are equipped to manage changes and make the labeling process run smoothly.
3.Standardizing processes across the organization with a common technology platform: Major pharmaceutical manufacturers and distributors are tying up with technologists to deploy a common technology platform and implement it across their locations. Companies that invest in business process uniformity will witness business improvement and growth. Quality issues often arise due to non-compliance of processes, undefined procedures, changing equipment and labels, etc. Many companies find that quality is impaired when processes vary from location to location. With the latest technology platforms, organizations can centrally assure that standardized practices are being followed across all locations.
4.Effective strategies to managing data: Non-compliance in the pharmaceutical industry is often a result of a poorly managed information loop. Systems supporting pharmaceutical manufacturing and distribution generate enormous data. When data is managed systematically, the right information is made available to appropriate users. The right system can notify regulatory changes, changes in formulations, or process variations. Every data point serves as a crucial piece of information that can guide users to be more proactive in conforming to business processes. Data management and analytics platforms are equipped to enable pharmaceutical companies to report anomalies as they occur. Dynamic reporting cumulatively helps in better quality compliance.
The Covid-19 pandemic has added impetus on pharmaceutical companies to adhere to regulations while working on therapeutics and vaccines at an unprecedented speed. Companies that leveraged the latest machine learning, analytics, and other IoT tools/ platforms, perform better.
Pharmaceutical companies are always looking for newer methods to balance quality compliance and productivity.
Johnson & Johnson, for instance, has a comprehensive quality management framework in place for continued focus on compliance and quality, amongst other essential parameters. The latest technologies and innovations back this framework.
All in all, reducing the risks pertaining to compliance issues in the pharmaceutical industry requires a well-planned and executed technological strategy. With the latest innovative platforms, companies can ensure adherence to quality compliance regulations in the industry.
Key Takeaways
- ERP, automation, data analytics, and machine learning are imperative in enabling pharma companies to mitigate the risks of non-compliance.
- To reduce the consequences of non-compliance, pharmaceutical companies need to strategize their technological moves.
Book a consultation to get started with our pharmaceutical compliance solutions.
References: Reducing the Risk of Noncompliance